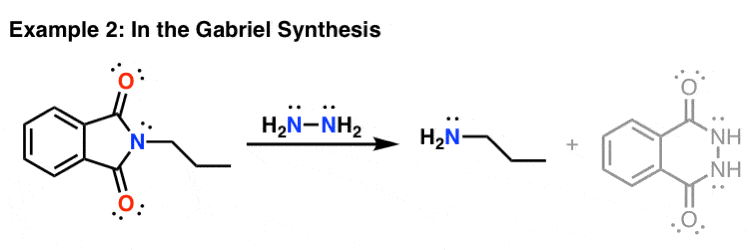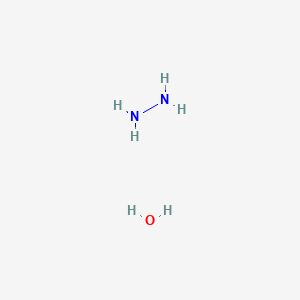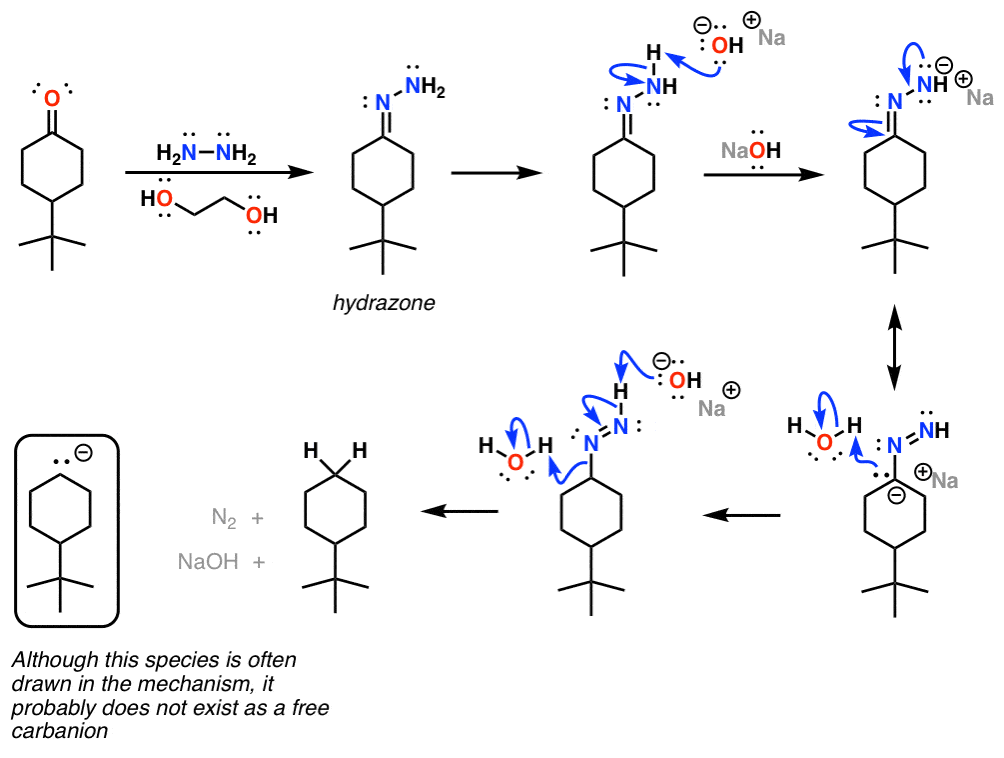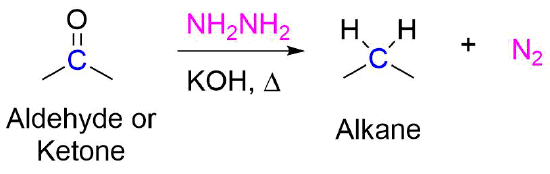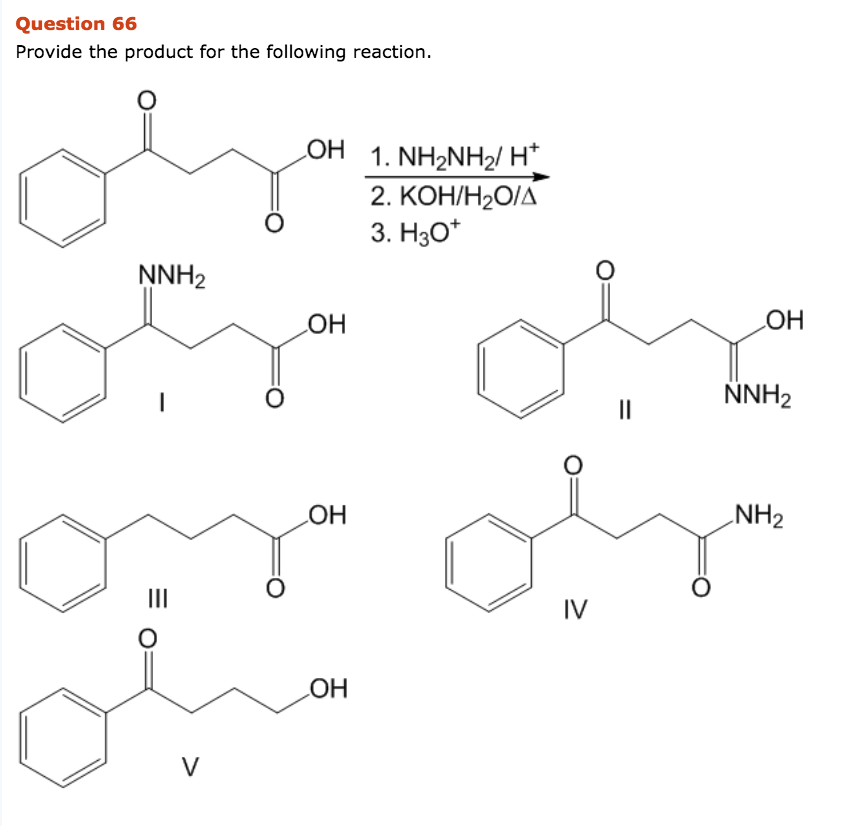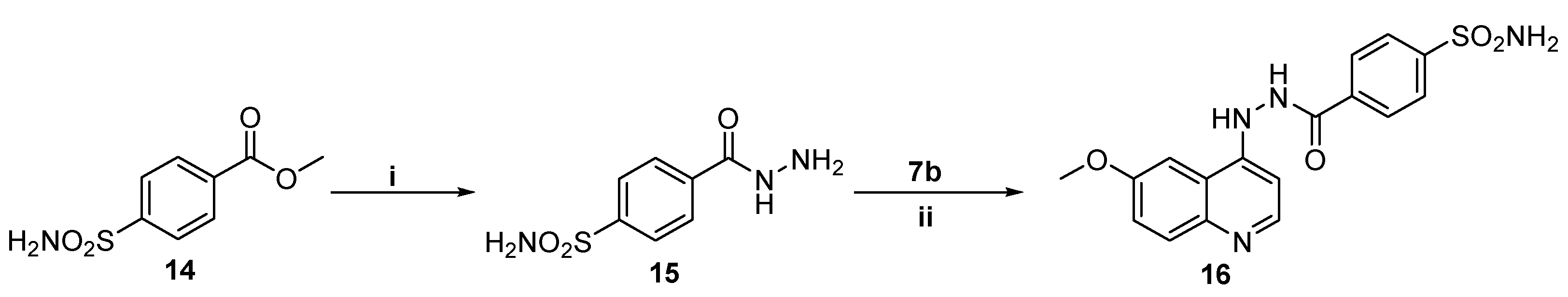
IJMS | Free Full-Text | Development of Novel Quinoline-Based Sulfonamides as Selective Cancer-Associated Carbonic Anhydrase Isoform IX Inhibitors

Conditions and reagents: (i) NH2NH2.H2O, EtOH, reflux, 6 h; (ii) DMF,... | Download Scientific Diagram

Scheme 1 | Synthesis, Urease Inhibition, Antioxidant, Antibacterial, and Molecular Docking Studies of 1,3,4-Oxadiazole Derivatives

Choice of Solvent (MeCN vs H2O) Decides Rate-Limiting Step in SNAr Aminolysis of 1-Fluoro-2,4-dinitrobenzene with Secondary Amines: Importance of Brønsted-Type Analysis in Acetonitrile | The Journal of Organic Chemistry
![5-Carbohydrazide and 5-carbonylazide of pyrazolo[3,4-b]pyridines as reactive intermediates in the synthesis of various heterocyclic derivatives - Ashraf A Aly, Talaat I El-Emary, Aboul-Fetouh E Mourad, Zainab Khallaf Alyan, Stefan Bräse, Martin Nieger, 5-Carbohydrazide and 5-carbonylazide of pyrazolo[3,4-b]pyridines as reactive intermediates in the synthesis of various heterocyclic derivatives - Ashraf A Aly, Talaat I El-Emary, Aboul-Fetouh E Mourad, Zainab Khallaf Alyan, Stefan Bräse, Martin Nieger,](https://journals.sagepub.com/cms/10.1177/1747519819861625/asset/images/large/10.1177_1747519819861625-fig2.jpeg)
5-Carbohydrazide and 5-carbonylazide of pyrazolo[3,4-b]pyridines as reactive intermediates in the synthesis of various heterocyclic derivatives - Ashraf A Aly, Talaat I El-Emary, Aboul-Fetouh E Mourad, Zainab Khallaf Alyan, Stefan Bräse, Martin Nieger,
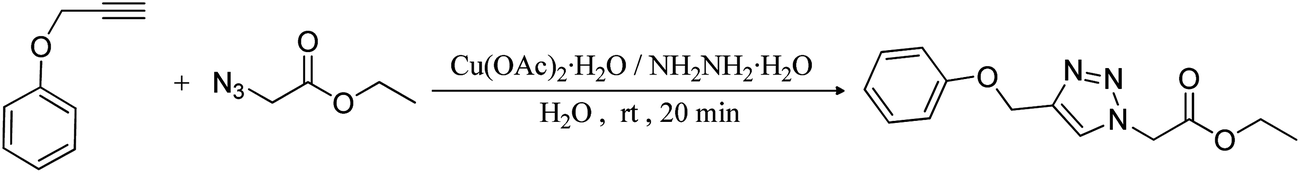
Cu(OAc) 2 ·H 2 O/NH 2 NH 2 ·H 2 O: an efficient catalyst system that in situ generates Cu 2 O nanoparticles and HOAc for Huisgen click reactions - RSC Advances (RSC Publishing) DOI:10.1039/C3RA45437A

Reagents and conditions: (a) EtOH, NaHCO3, reflux, 24h; (b) NH2NH2·H2O,... | Download Scientific Diagram
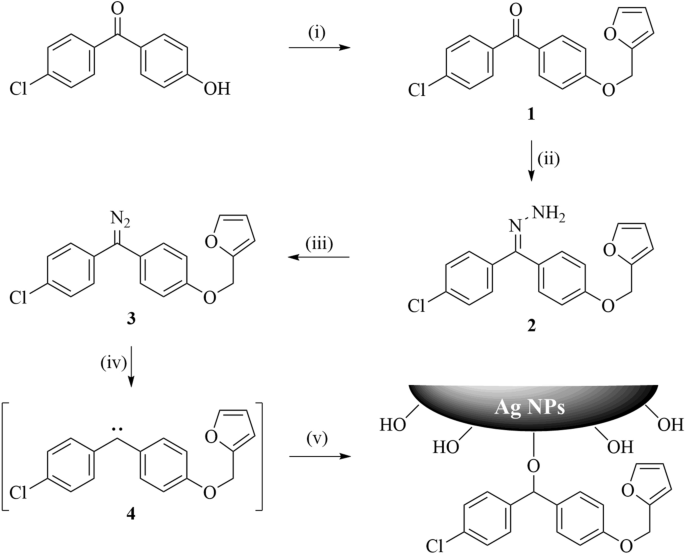
Carbene modification and reversible crosslinking of silver nanoparticles for controlled antibacterial activity | Scientific Reports
![Scheme 1, [(i) NH2NH2·H2O, EtOH (ii) PhC(O)CO2Et,...]. - Probe Reports from the NIH Molecular Libraries Program - NCBI Bookshelf Scheme 1, [(i) NH2NH2·H2O, EtOH (ii) PhC(O)CO2Et,...]. - Probe Reports from the NIH Molecular Libraries Program - NCBI Bookshelf](https://www.ncbi.nlm.nih.gov/books/NBK143197/bin/ml228f12.jpg)
Scheme 1, [(i) NH2NH2·H2O, EtOH (ii) PhC(O)CO2Et,...]. - Probe Reports from the NIH Molecular Libraries Program - NCBI Bookshelf
Discovery of Novel Orally Active Tetrahydro-Naphthyl-N-Acylhydrazones with In Vivo Anti-TNF-α Effect and Remarkable Anti-Inflammatory Properties | PLOS ONE