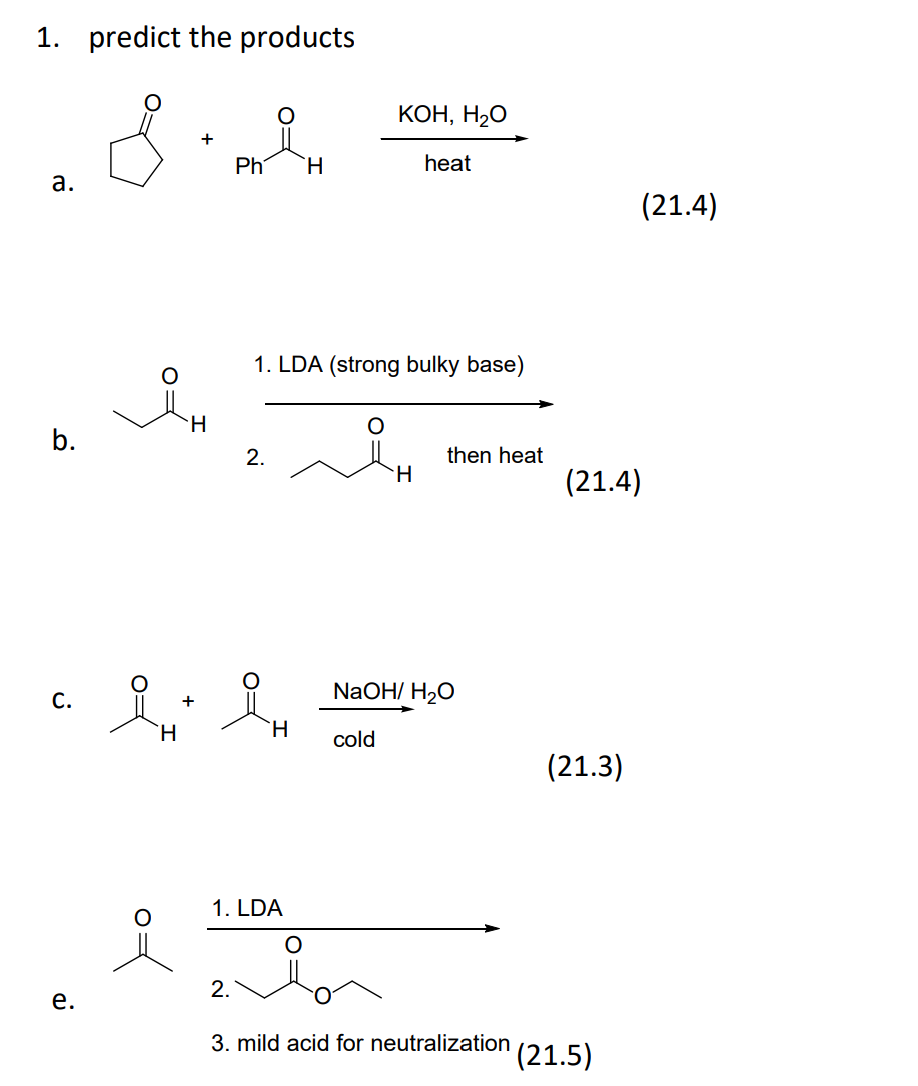
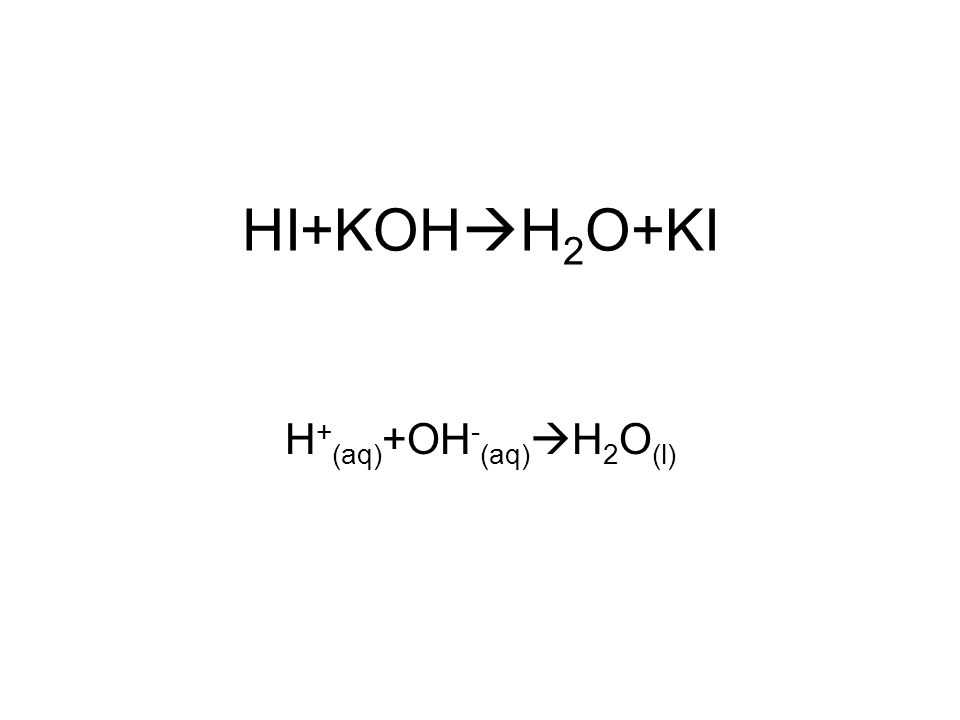
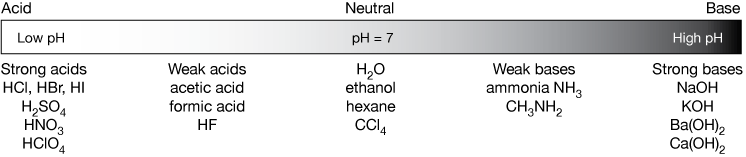
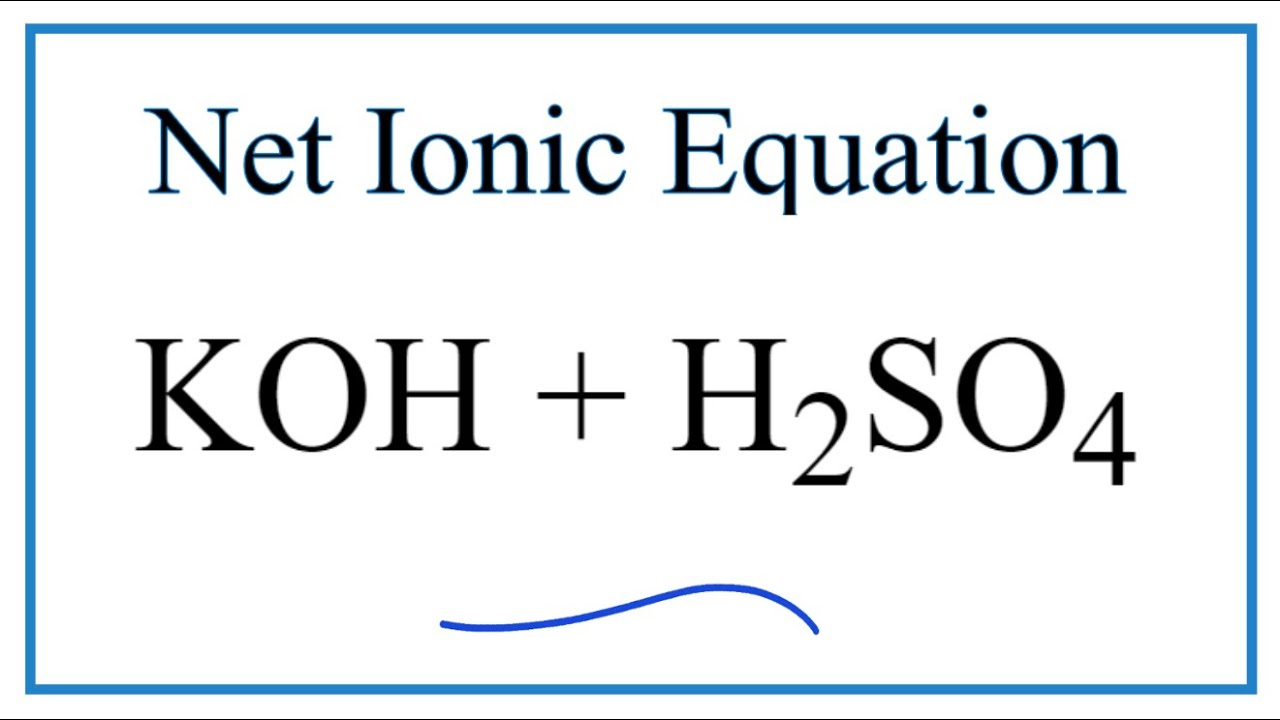Write the neutralization reaction for the following acid and base: HCl_{(aq)} and KOH_{(aq)}. | Homework.Study.com
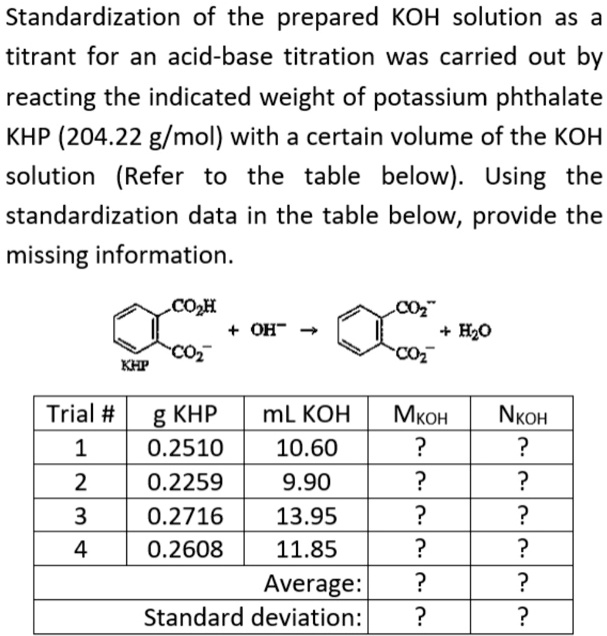
SOLVED: Standardization of the prepared KOH solution as titrant for an acid- base titration was carried out by reacting the indicated weight of potassium phthalate KHP (204.22 g/mol) with a certain volume of

SOLVED: Potassium hydroxide dissociates in water to produce hydroxide ions KOH(s) +HzO() Kt(aq) + OH (aq) Given the information above, how is potassium hydroxide categorized? Both a Bronsted-Lowry base ad an Arrhenius