
Measured (fitted) diffusion resistances in H2O/H2 and CO2/CO mixtures... | Download Scientific Diagram

Consider the following reaction at certain temperature: H2O(g)+CO2(g) equilibrium to H2(g)+CO2(g) Some molecules of H2O and CO are placed in a 1.0 L container as shown below. When equilibrium is reached, how
3g of H2 reacts with 29 g of O2 to give H2O.Find i) Limiting reagent ii)Max amount of H2O formed iii) Amount of reactants which remains unreacted.
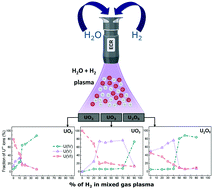
Mixed H2O/H2 plasma-induced redox reactions of thin uranium oxide films under UHV conditions - Dalton Transactions (RSC Publishing)
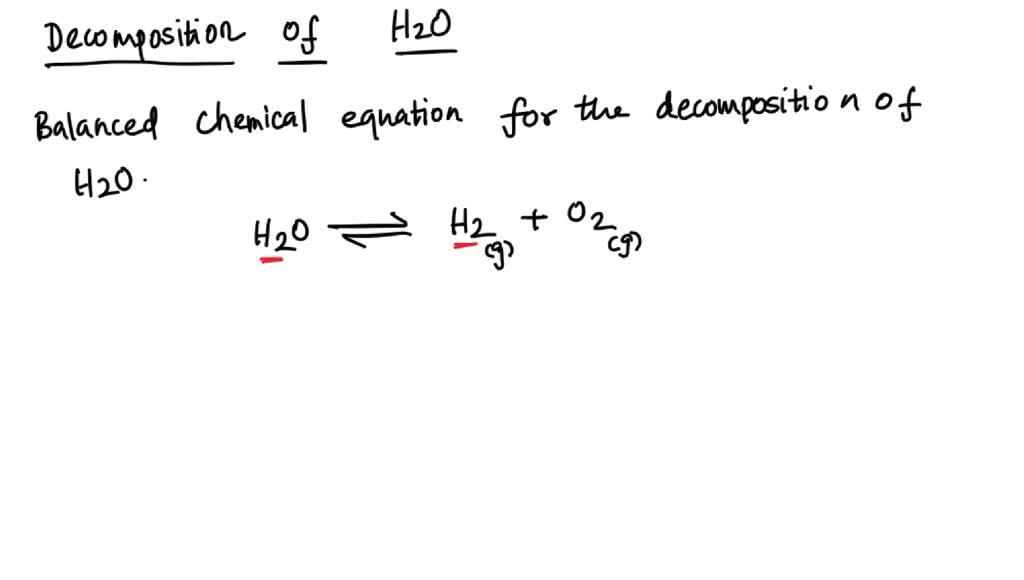
SOLVED: The decomposition of water into hydrogen gas H2 and oxygen gas O2 can be modeled by the balanced chemical equation A) H2 + O2 → H2O B) H2O → H2 +

calculate the equilibrium constant of H2 + O2 gives us H2O + CEO at 13957 if the equilibrium constant 135 - Chemistry - Equilibrium - 13886927 | Meritnation.com

n mole each of H2O, H2 and O2 are mixed at a suitable high temperature to attain the equilibrium 2H2O 2H2 + O2 . If y mole of H2O are dissociated and
Kp for the reaction CO2 + H2 =CO + H2O is found to be 16 at a given temperature. Originally equal number of moles of H2 and CO2 were placed in the



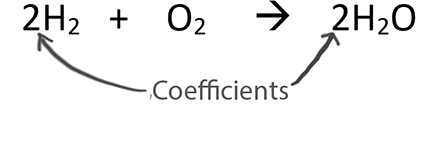



![PDF] Initiation in H2/O2: Rate constants for H2+O2→H+HO2 at high temperature | Semantic Scholar PDF] Initiation in H2/O2: Rate constants for H2+O2→H+HO2 at high temperature | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/59651c1a79d5be73d45e3b492f6f4396965dd05f/5-Table1-1.png)
