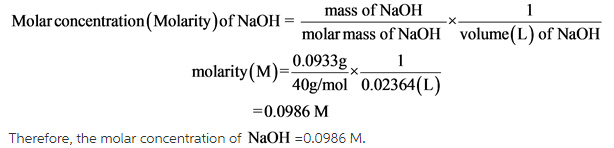Calculate the amount of oxalic acid (H2C2O4.2H2O) required to obtain 250 mL of deci-molar solution. - Sarthaks eConnect | Largest Online Education Community
What mass of oxalic acid dihydate, H2C2O4•2H2O, is needed to make a 0.498 M solution of oxalic acid in a 250.0 mL volumetric flask? - Quora

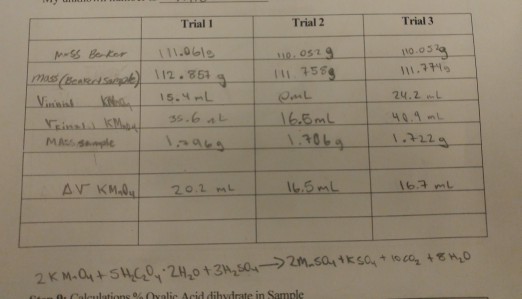
OneClass: how do i do question #4? Calculate the molar mass of oxalic acid dihydrate H_2C_2O_4 2H_2O....
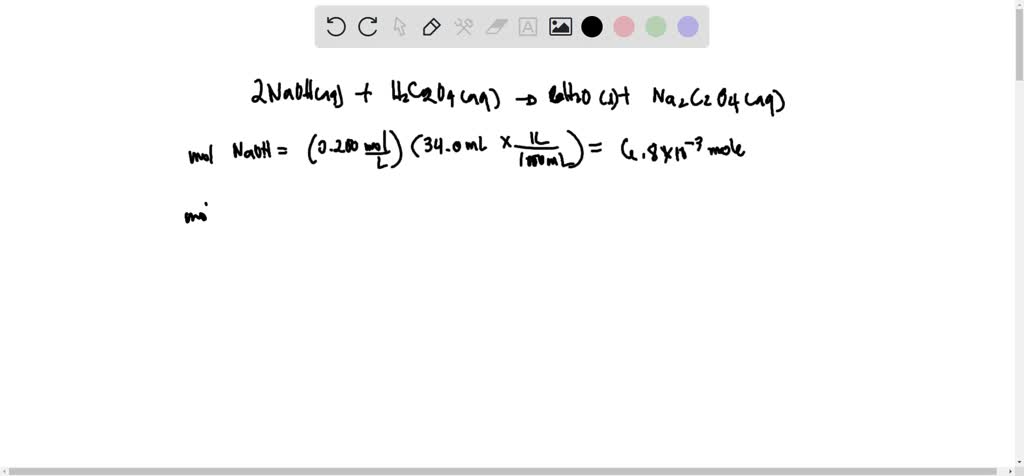
SOLVED: 2 NaOH(aq) + H2C2O4 = 2H2O(s) Na2C2O4(aq) + 4 H2O(l) Calculate the molarity of an oxalic acid solution if it takes 34.0 mL of 0.200 M NaOH solution to consume the

What mass of oxalic acid dihydate, H2C2O4•2H2O, is needed to make a 0.498 M solution of oxalic acid in a 250.0 mL volumetric flask? - Quora
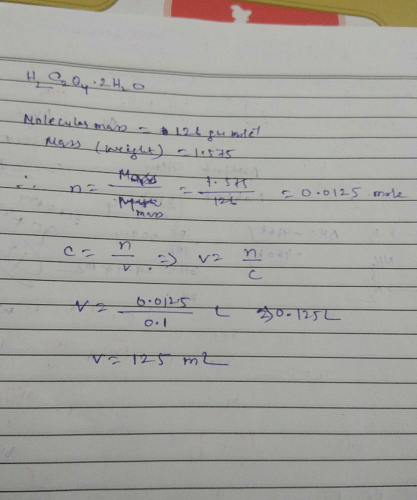
Volume (mL) of H2C2O4. 2H2O solution to prepare 0.10 M from 1.575g of it is ?Correct answer is '125'. Can you explain this answer? | EduRev Chemistry Question
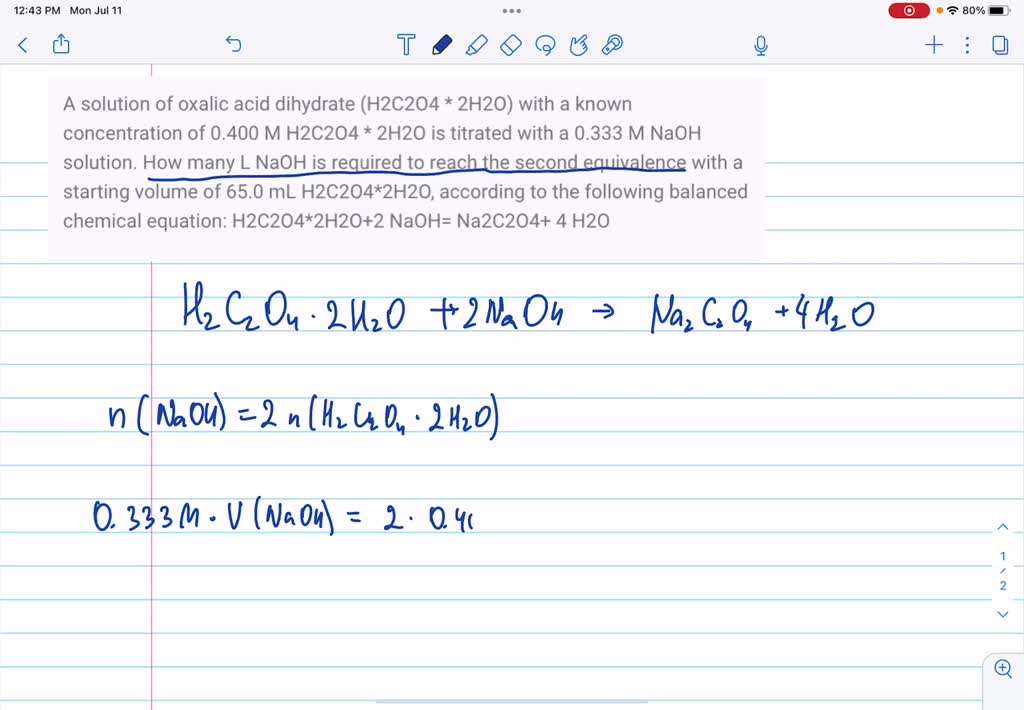
SOLVED: A solution of oxalic acid dihydrate (H2C2O4 * 2H2O) with a known concentration of 0.400 M H2C2O4 * 2H2O is titrated with a 0.333 M NaOH solution. How many L NaOH

High Quality Oxalic Acid H2c2o4.2h2o,99.6% Min Purity - Buy Oxalic Acid H2c2o4.2h2o,Oxalic Acid C2h2o4.2h2o,Oxalic Acid H2c2o4 2 H2o Product on Alibaba.com