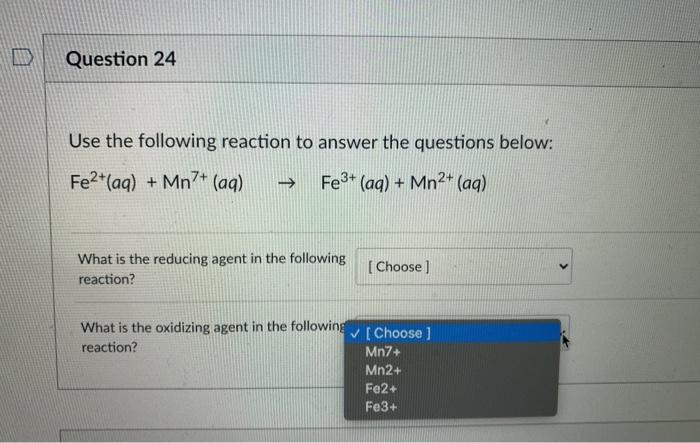
Aqueous-Phase Differentiation and Speciation of Fe3+ and Fe2+ Using Water-Stable Photoluminescent Lanthanide-Based Metal–Organic Framework | ACS Applied Nano Materials

Color online) Energetic positions of Fe (Fe1, Fe2, and Fe3) and B (B... | Download Scientific Diagram

Exploring selective recognition between Fe2+, Fe3+ and their implementation in bio-imaging: A combined spectroscopic and theoretical investigation - ScienceDirect
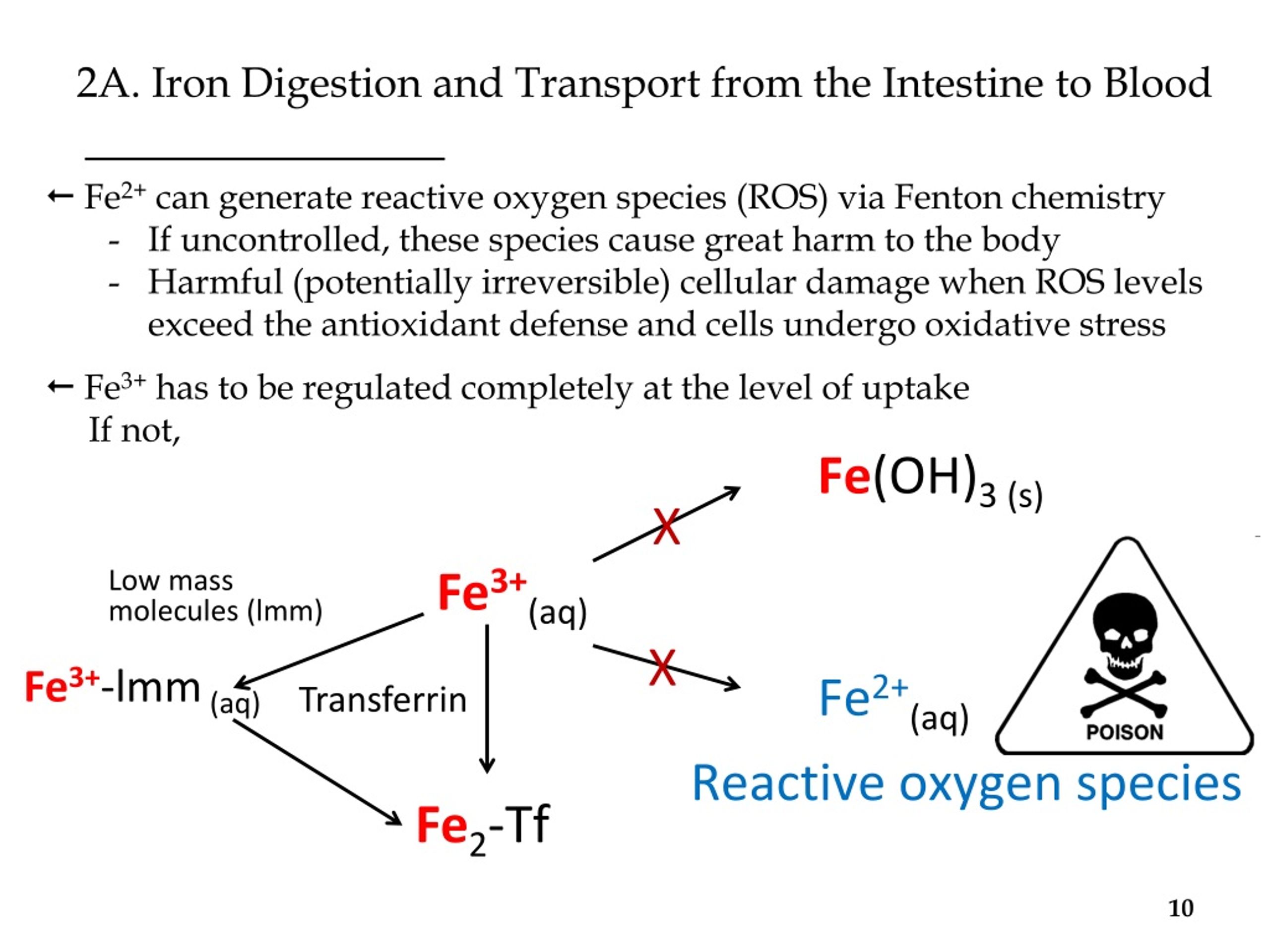
PPT - Iron (Fe 2+ /Fe 3+ ) Transport and Trafficking in Mammals Bertini et al Ch. 5 and 8 PowerPoint Presentation - ID:432903

SOLVED: Consider the following voltaic cell: Pt(s)IFe2+ (aq) , Fe3+(aq) Ag" (aq)Ag(s) Identify the half-reaction that occurs at the anode Select one: a. Fe3- (aq) + e Fe2- (aq) b. Pt(s) +

Comparison of Fe 3 + /Fe 2 + ratios determined for standard basaltic... | Download Scientific Diagram
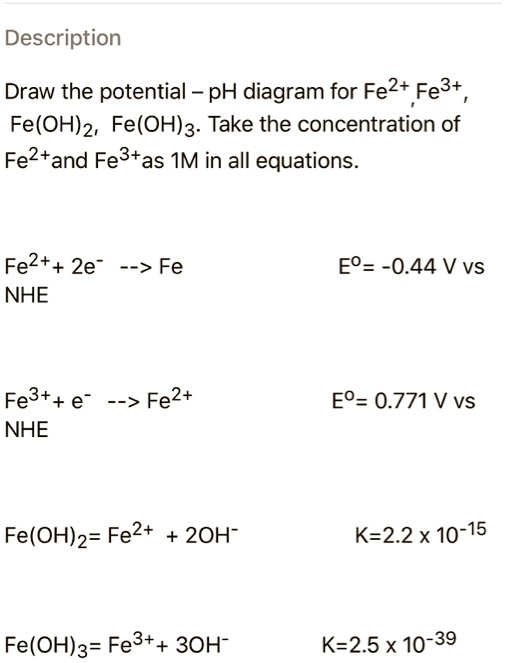
SOLVED: Description Draw the potential - pH diagram for Fe2+ Fe3+ Fe(OHJz, Fe(OH)g. Take the concentration of Fe2+and Fe3+as IM in all equations. Fe2++ 2e –> Fe NHE EO= -0.44 V vs

Sketch the voltaic cell containing Zn|Zn2+ and Fe2+|Fe3+ half-cells. Calculate the Ecell. Be sure to label everything. Hint: There has to be a solid state support. | Homework.Study.com

Homogeneous photocatalytic Fe3+/Fe2+ redox cycle for simultaneous Cr(VI) reduction and organic pollutant oxidation: Roles of hydroxyl radical and degradation intermediates - ScienceDirect

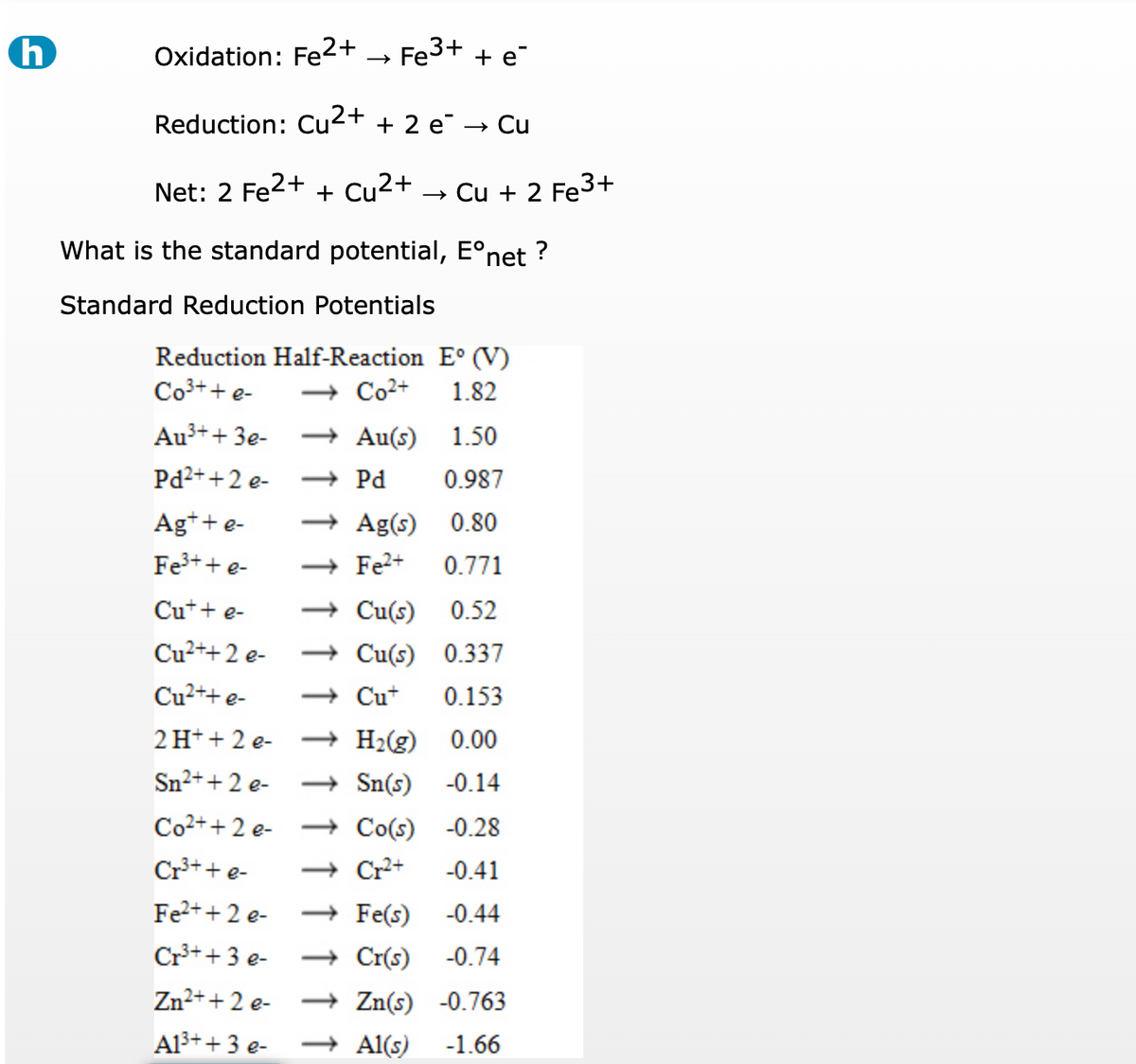
.PNG)