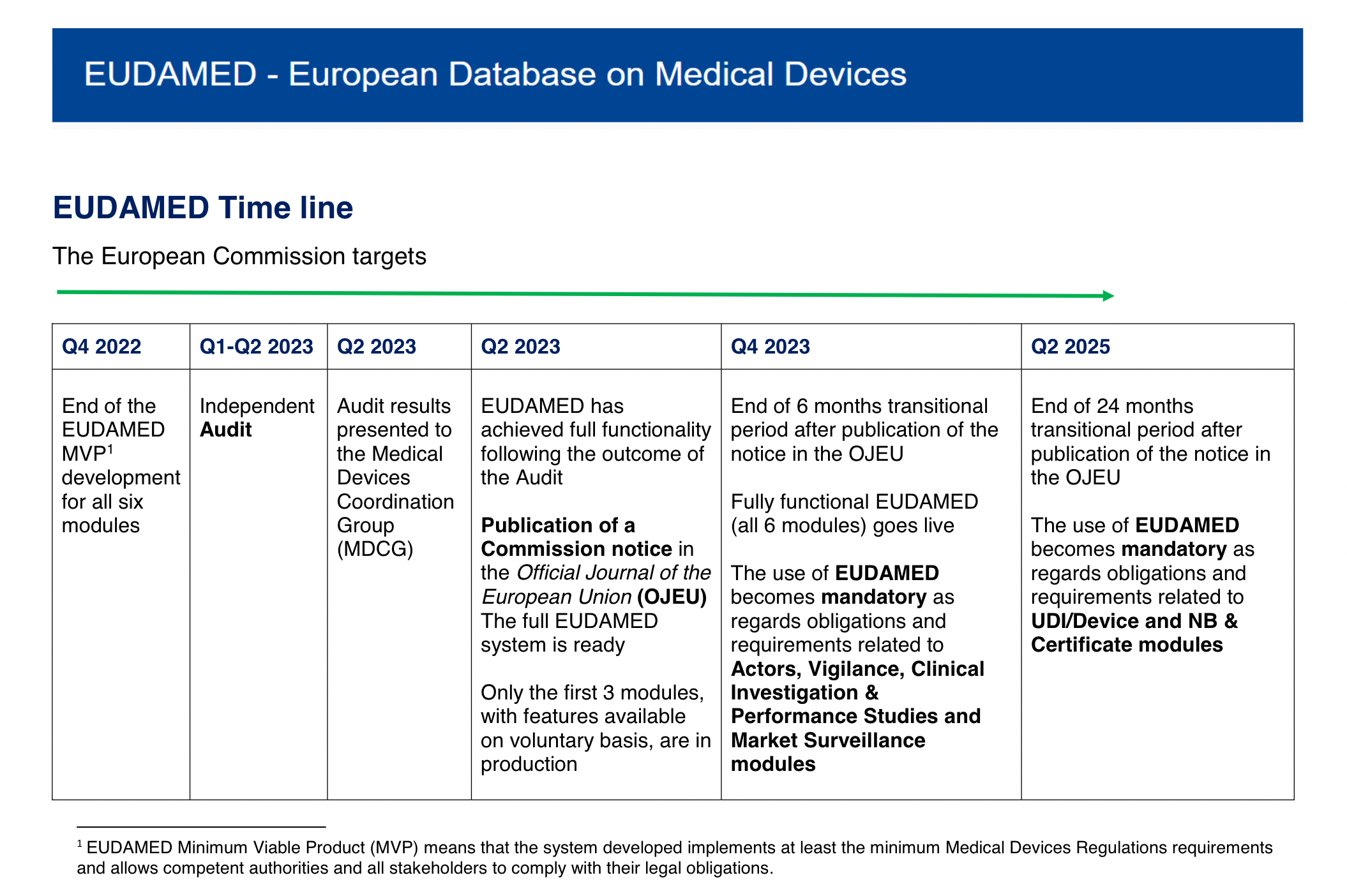
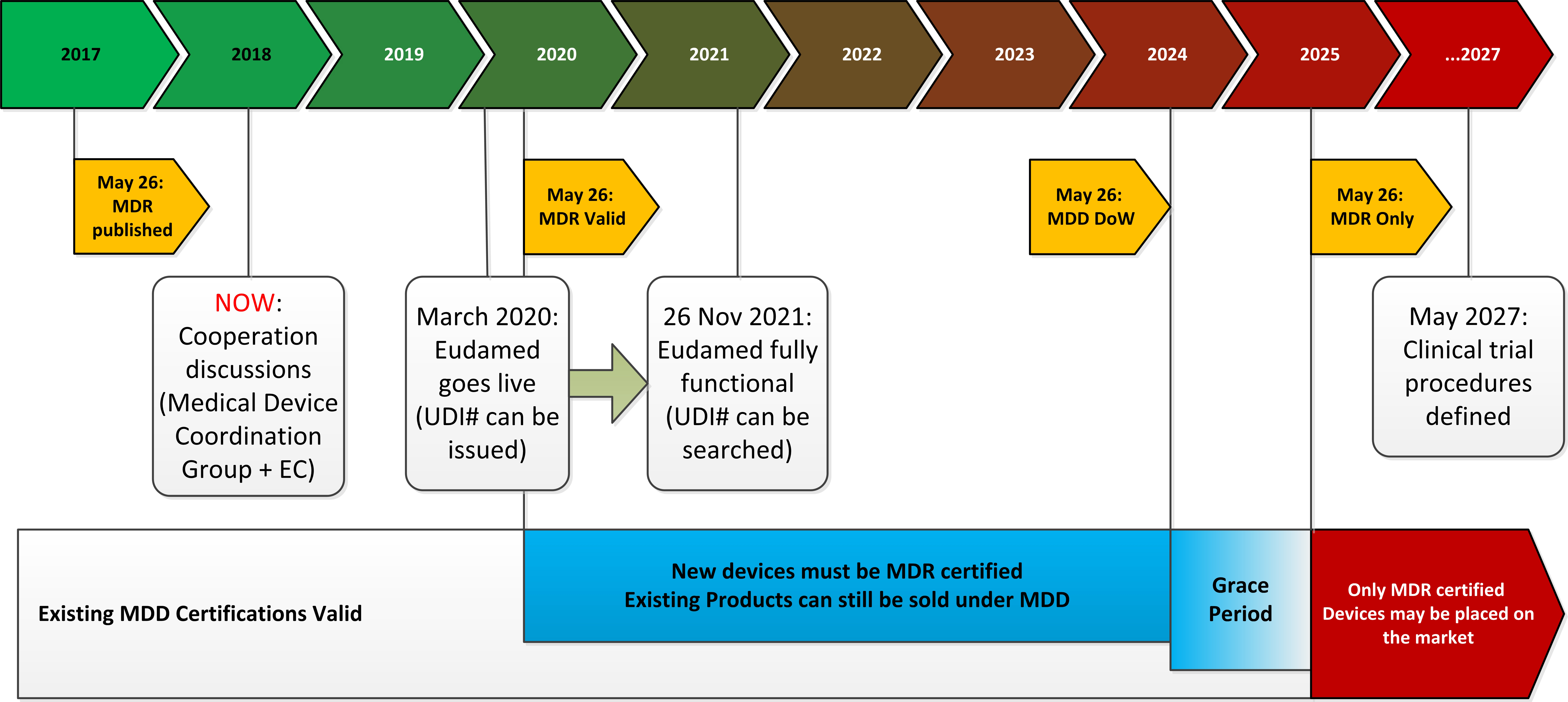
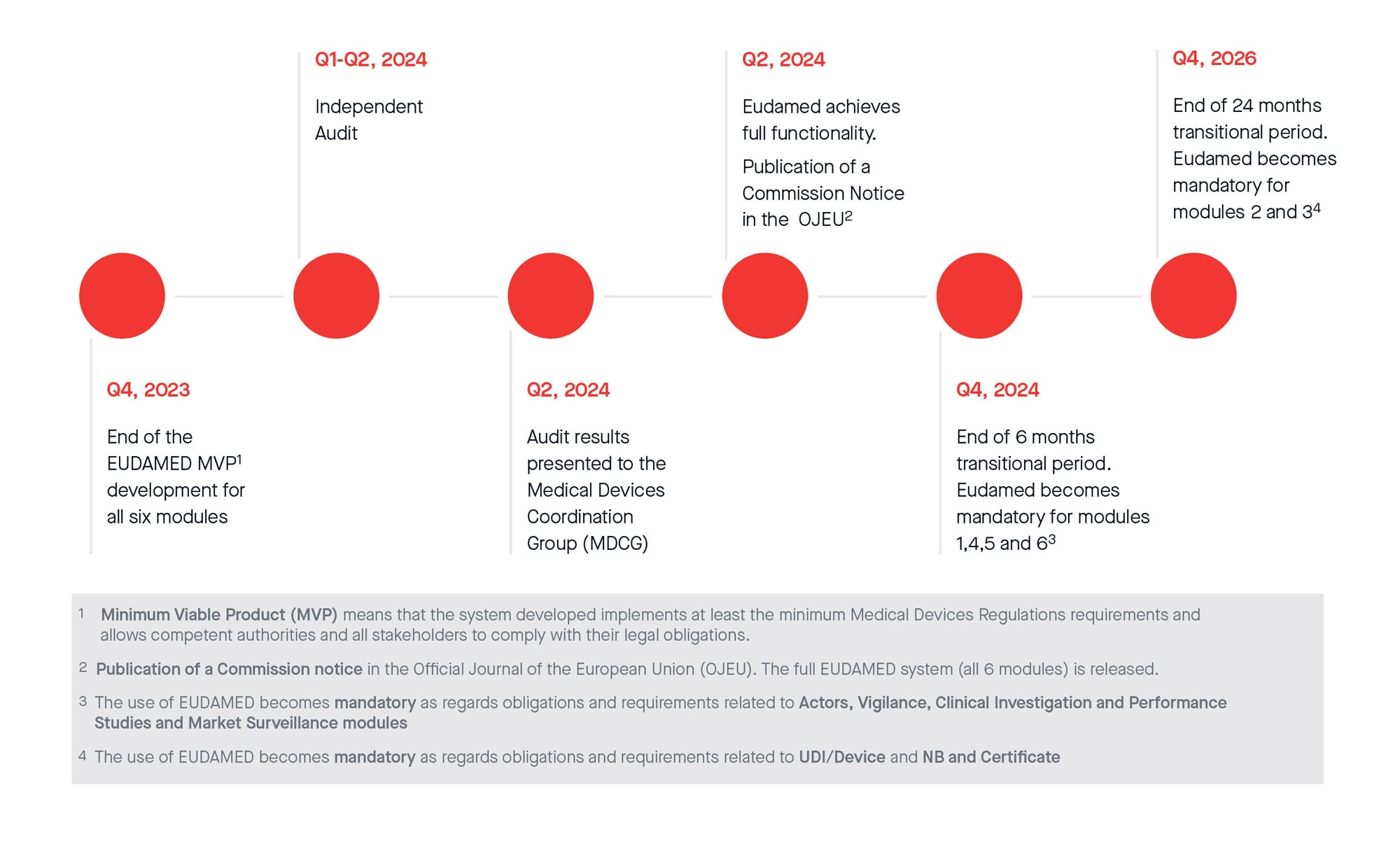
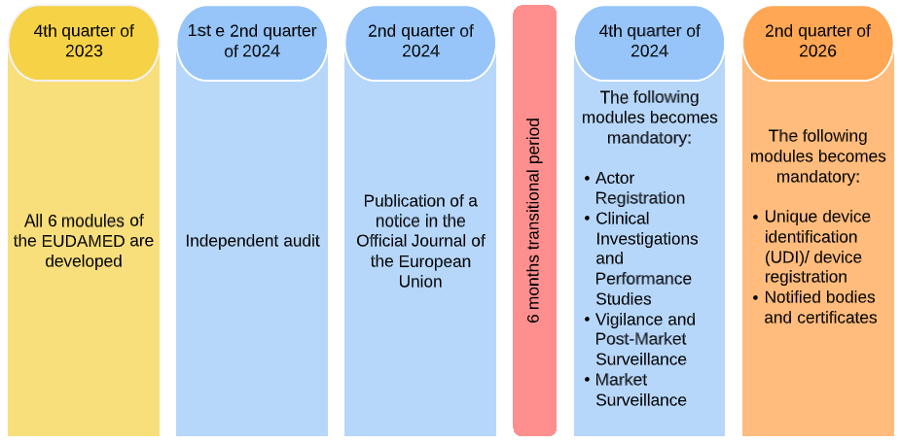
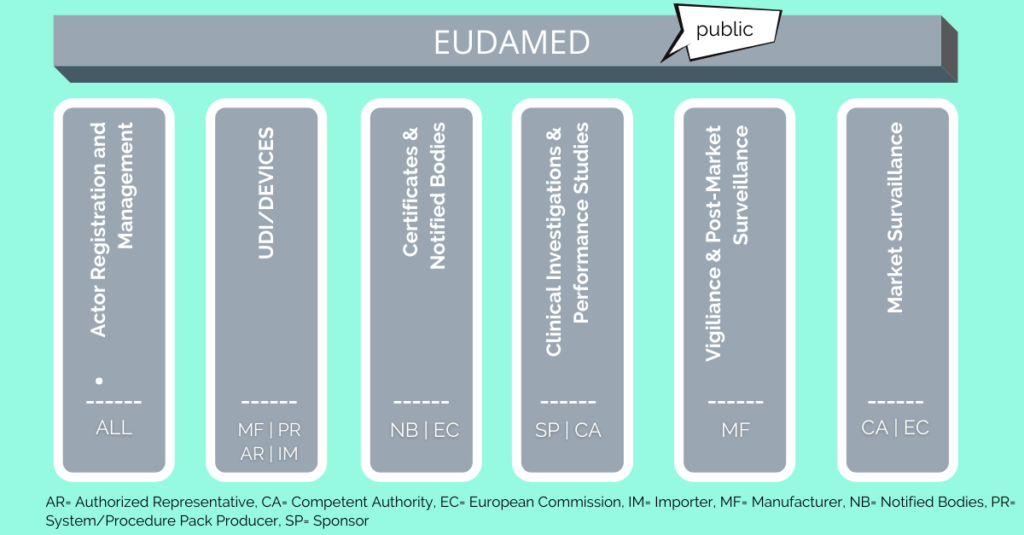
New European medical device regulation: How the French ecosystem should seize the opportunity of the EUDAMED and the UDI system, while overcoming the constraints thereof - ScienceDirect

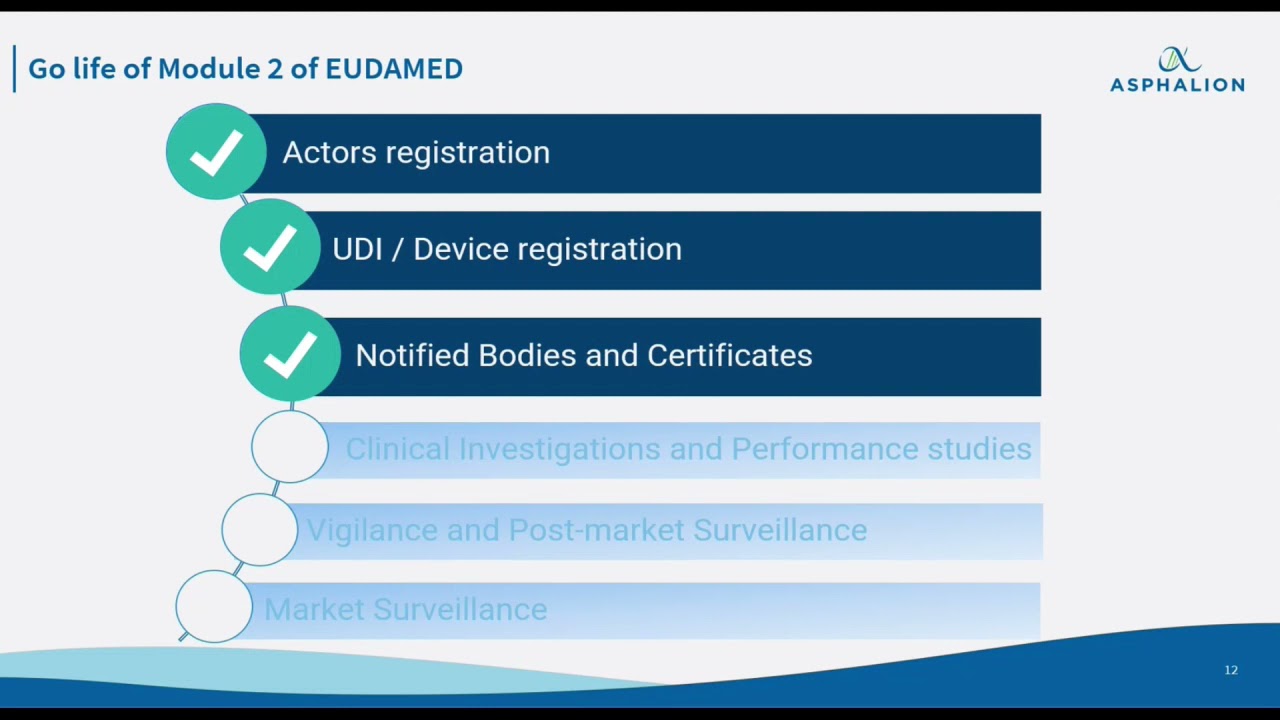
UDI & EUDAMED Explained under EU MDR - Clinical, Regulatory & Automation solutions for Life Sciences