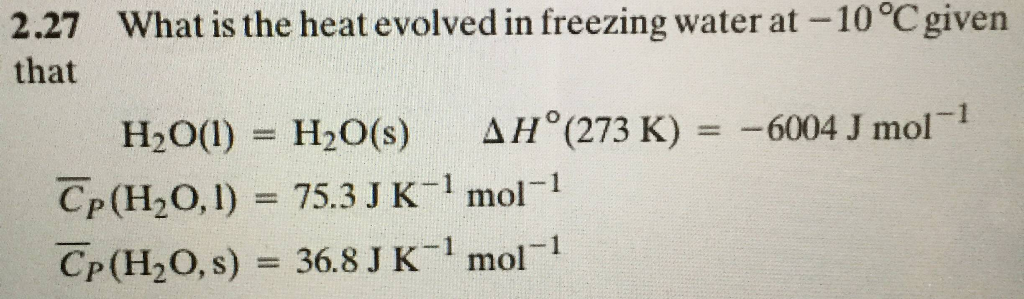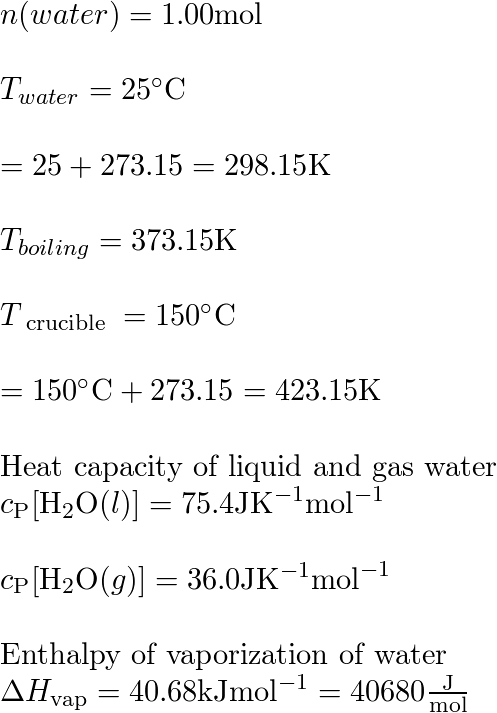![Cp∗Rh(bpy)(H2O)]2+: a versatile tool for efficient and non-enzymatic regeneration of nicotinamide and flavin coenzymes - ScienceDirect Cp∗Rh(bpy)(H2O)]2+: a versatile tool for efficient and non-enzymatic regeneration of nicotinamide and flavin coenzymes - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S1381117702001649-gr1.gif)
Cp∗Rh(bpy)(H2O)]2+: a versatile tool for efficient and non-enzymatic regeneration of nicotinamide and flavin coenzymes - ScienceDirect

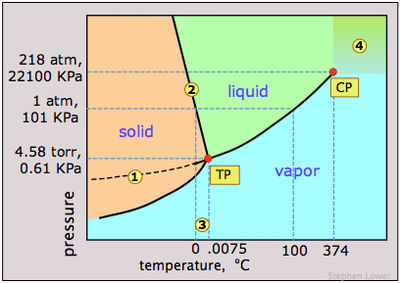
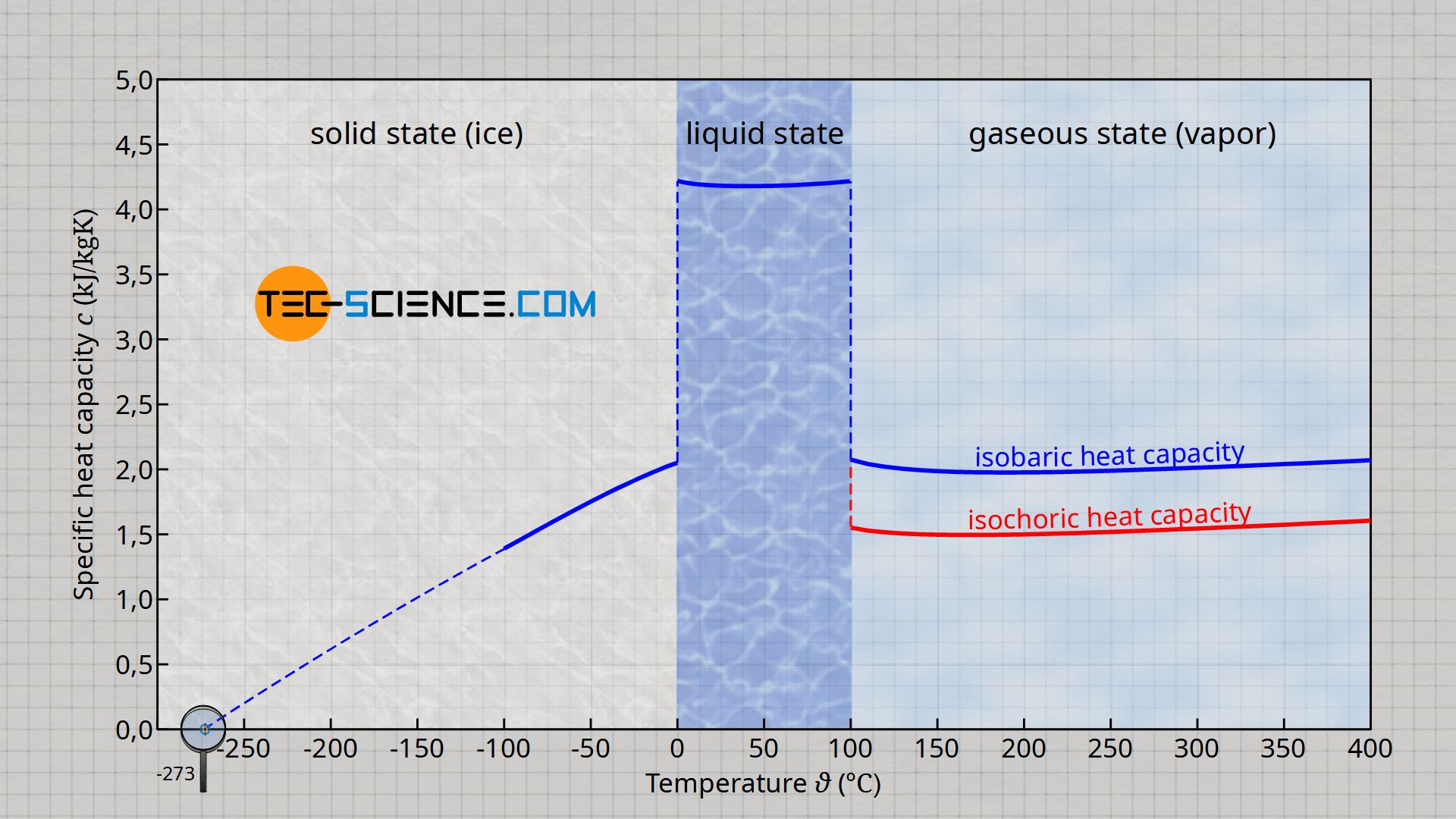
Calcualte the enthalpy change on freezing of 1.0 mole of water at 10.0^(@)C to ice at -10^(@)C. ... - YouTube
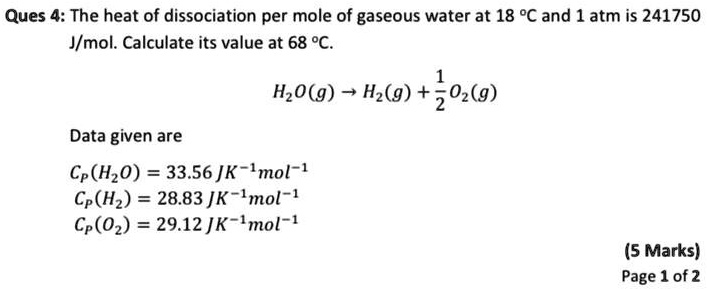
SOLVED: Ques 4: The heat of dissociation per mole of gaseous water at 18 %C and 1 atm is 241750 Jmol. Calculate its value at 68 %. HzO(g) Hz(g) + 202(9) Data

The System CaCl2–H2O: Thermodynamic Modeling and Flow Calorimetry Experiments at Elevated Temperatures and Pressures | Journal of Chemical & Engineering Data

H2O International SHH-CP-IL Handheld Shower Inline Filter with Chlorine Removal System Chrome Finish - Hand Held Showerheads - Amazon.com

The enthalpy change for a reaction at equilibrium is - 20.5 kJ mol ^-1 . Then the entropy change for this equilibrium at 410 K is:
![Calculate the enthalpy change of freezing of 1.0 mol of water at 10^(@)C to ice at -10^(@)C, Delta(fus)H=6.03 kJ mol^(-1) at 0^(@)C. C(P)[H(2)O(l)]=75.3 J mol^(-1) K^(-1) C(P)[H(2)O(s)]=36.8 J mol^(-1) K^(-1) Calculate the enthalpy change of freezing of 1.0 mol of water at 10^(@)C to ice at -10^(@)C, Delta(fus)H=6.03 kJ mol^(-1) at 0^(@)C. C(P)[H(2)O(l)]=75.3 J mol^(-1) K^(-1) C(P)[H(2)O(s)]=36.8 J mol^(-1) K^(-1)](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/34965050_web.png)