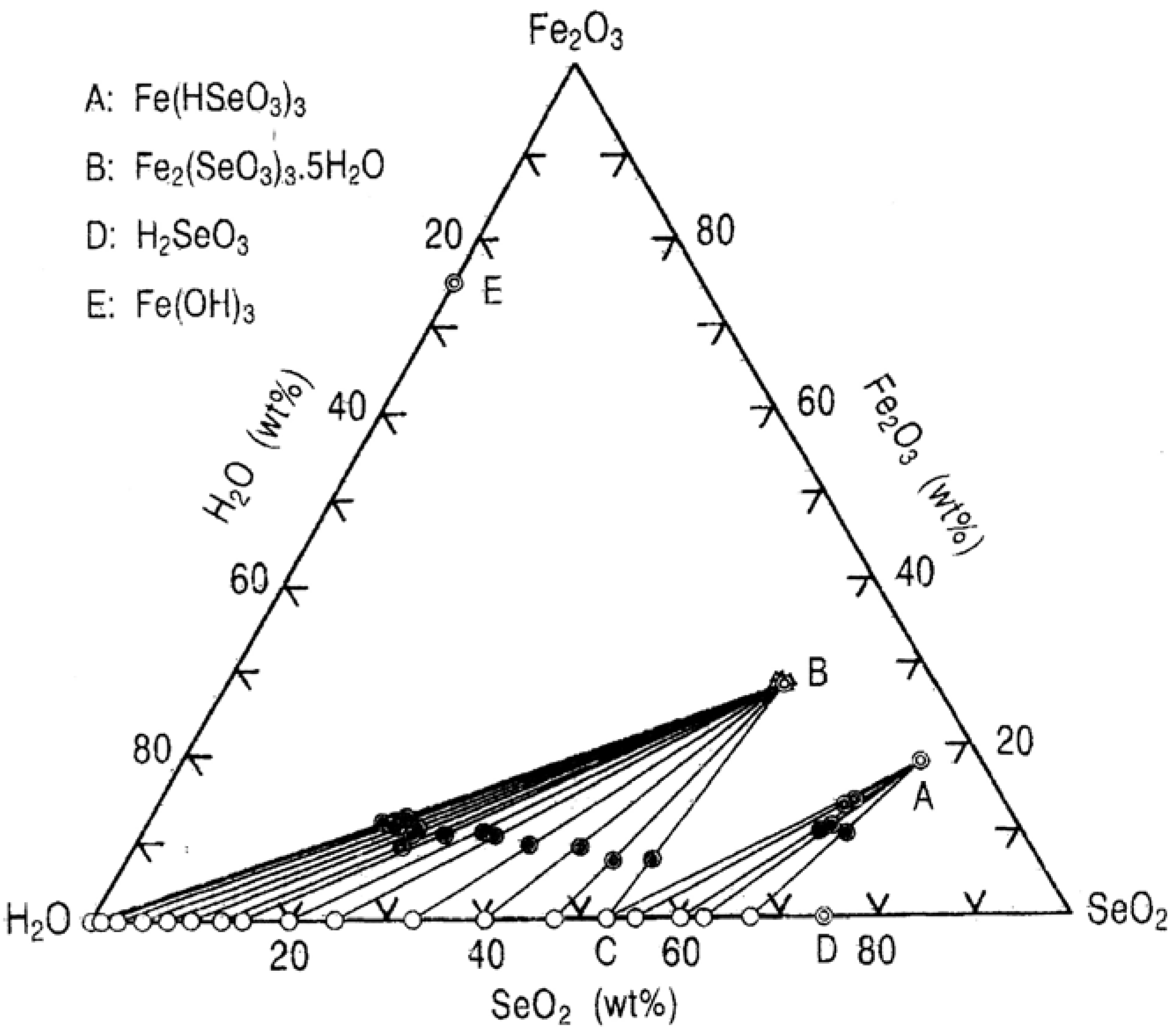
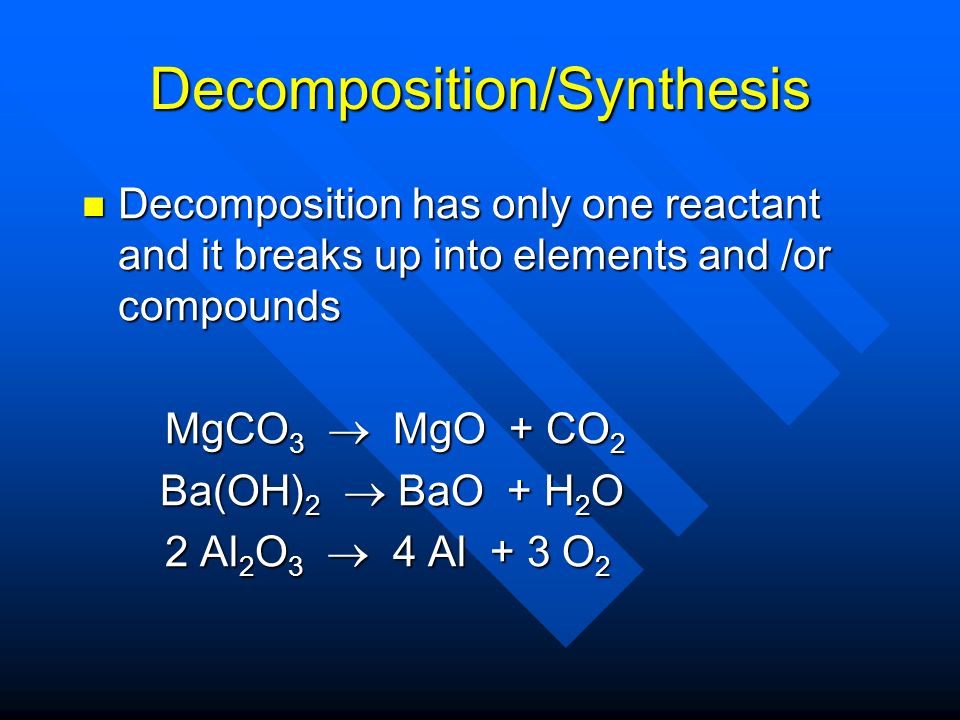
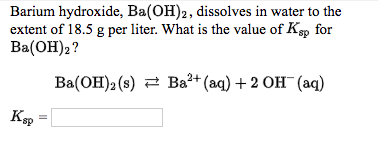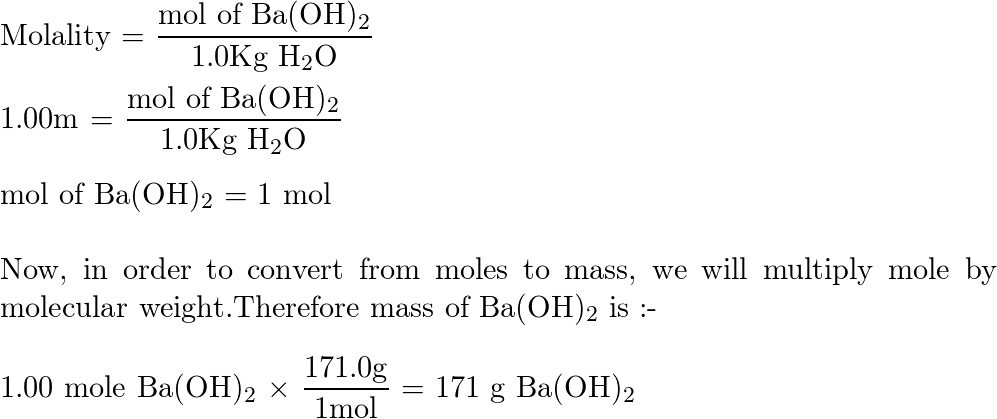рассчитайте по термохимическому уравнению BaO+H2O=Ba(OH)2+73 кДж сколько выделяется энергии - Школьные Знания.com

The solubility of Ba (OH)2 . 8H2O in water ar 288K is 5.6g per 100g of water. What is the molality of the hydroxide ions in saturated solution of Ba (OH)2 .
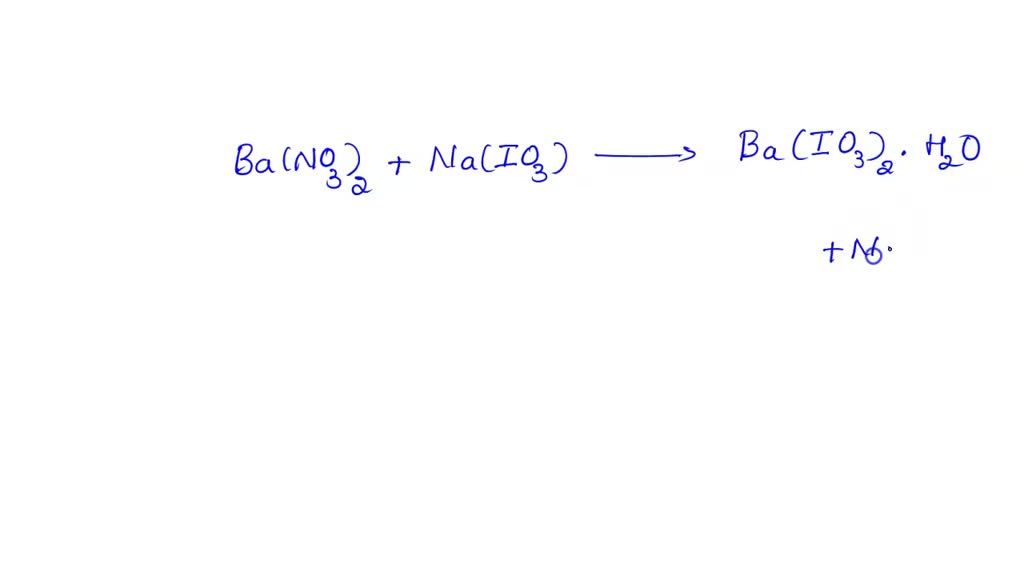
SOLVED: 1) Student synthesized 6.895 g of Ba(IO3)2 x H2O, by adding 30 mL of 0.5912 M Ba(NO3)2 to 50 mL of 0.9004 M NaIO3. a. Write a balanced molecular equation for
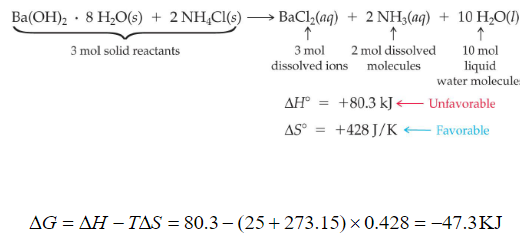
BALANCE THIS GIVEN EQUATION BY THE HELP OF THE ALTERNATE WAY OF BALANCING NOT THE TRADITIONAL WAY BY SHOWING THE APPROPRIATE STEPS ELABORATELY. EQN : Ba(OH)2 + NH4Cl —> BaCl2 + NH3 + H2O

Unexpected size distribution of Ba(H2O)n clusters: why is the intensity of the Ba(H2O)1 cluster anomalously low? - Physical Chemistry Chemical Physics (RSC Publishing)