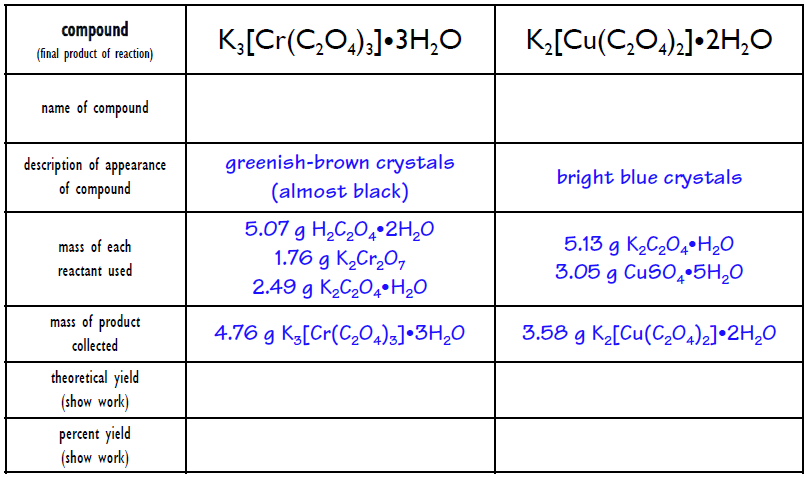
![Copper(II) Sulfate Pentahydrate Pentahydrate (Blue Vitriol) [CuSO4.5H2O] Molecular Weight Calculation - Laboratory Notes Copper(II) Sulfate Pentahydrate Pentahydrate (Blue Vitriol) [CuSO4.5H2O] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2022/11/copper-sulfate-pentahydrate-molecular-weight-calculation.jpg)
Copper(II) Sulfate Pentahydrate Pentahydrate (Blue Vitriol) [CuSO4.5H2O] Molecular Weight Calculation - Laboratory Notes

SOLVED: Molecules of CuS04.5H2O or C u 04 dot 5 H20 in the sample? 2 moles € u S 04 dot 5 H2O X =3 "Question mark" moles Cu 04 dot 5

Under what vapour pressure of moisture in atmospheric conditions CuSO4· 5H2O will be efflorescent if CuSO4· 5H2O (s) CuSO4· 3H2O + 2H2O (g) ; Kp = 62.73 (mm)^2